Atom:-
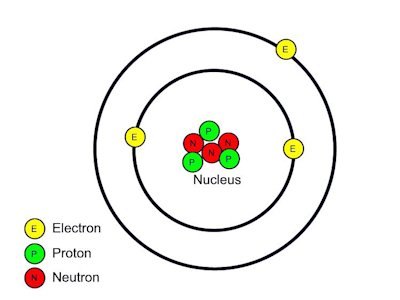
Problem :1 Name the smallest particle of matter which can't further divisible?
Ans:-Atom
problem 2:Who discover electron,proton and neutron?
Ans:-Electron discovered by:-J.J.Thomson in 1897
Proton discovered by:-Goldstein in 1886
Neutron discovered by:-Chadwick in 1932.
Problem 3: What is charge on electron,proton and neutron?
Ans:-Charge on Electron:-Negative charge which is=1.602*10-19coulombs(-1)
Charge on Proton:-positive charge which is=1.602*10-19coulombs(+1)
Charge on Neutron:-No charge
Problem 4: What is the order of radius of nucleus and atom?
Ans:- Radius of Atom:- 1*10-8cm
Radius of Nucleus:- 1*10-12cm
Problem 5: Number of proton in nucleus is called? Who discover that?
Ans:-Atomic number.It is discovered by Mosley.
Problem 6: What is Atomic mass?
Ans:-Sum of numbers of proton and neutron in nucleus.
Problem 7:Atoms of different elements whose mass number same and atomic number different is called?Give example.
Ans:-Isobar.Ex. 18Ar40,19K40
Problem 8:Atoms of different elements whose nuclei contain same number of neutron are called ?Give example.
Ans:-Isotones.Ex.14Si30,,15P31
Problem 9: Atom is summarized in how many quantum numbers?
Ans:- 4
1. Principle quantum number
2.Azimuthal quantum number.
3. Magnetic quantum number.
4. Spin quantum number
Problem 10: What is Aufbau principle?
Ans:- Electron enters an orbital of lowest energy.
No comments:
Post a Comment